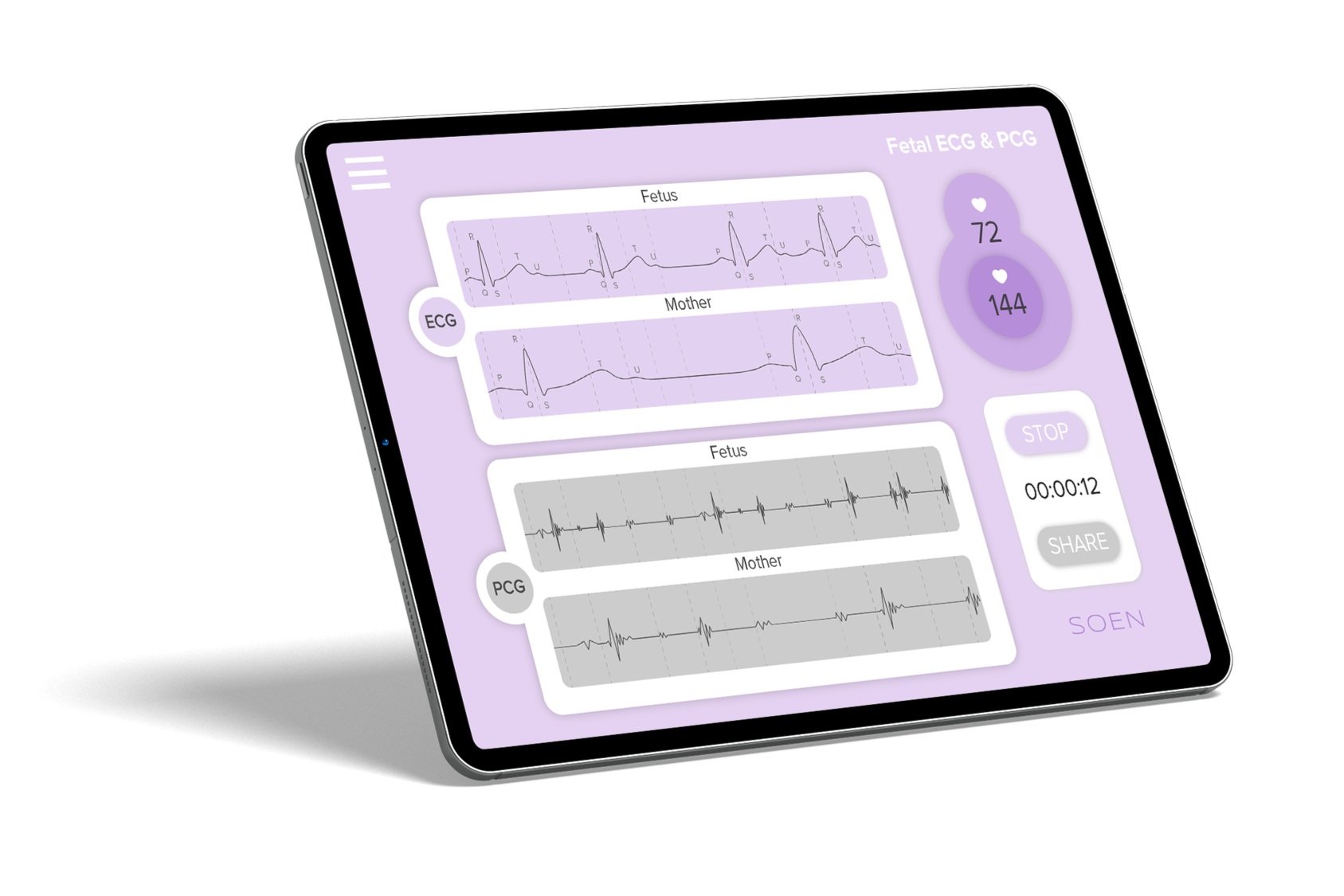
Prevention through data
Axalton Med Tech offers solutions to global healthcare challenges in three distinctive, yet comprehensive activities.
1.
User-Friendly, Human Centered Medical Grade Devices:
2.
Telemedicine Services
Health monitoring and therapy support for patients & physicians
3.
Axalton Data/AI Center
AI powered data evaluation, e-diagnostics & data management
Storing, managing, and evaluating data coming from medical sensors
Managing data based on proper authentication and process description of the telemedicine services
Evaluating the data with our medical expert team and AI
Knowledge and algorithm development platform for telemedicine services
AI development center
Selling data - under the highest legal and ethical standards
The accumulated data coming from the sensors and from the telemedicine services from real patients is of high value itself.
It can be utilized in developing the knowledge of our medical team and partner physicians, in AI self-learning to validate and enhance our evaluation algorithms, which can further be sold to health service providers, R&D partners and pharmaceutical companies.
In selling health data, we want to follow the highest legal and ethical standards, with which we intend to contribute to the development of science and health technology, as well as to generate new telemedicine market segments, where Axalton Med Tech aims to have a visible market share.
Data products of the Axalton Med tech solution
Selling aggregated and anonymized data removing any personally identifiable information (PII) from the data, making it impossible to identify individuals. Aggregated and anonymized data can provide valuable insights for research, public health, or healthcare analytics purposes without compromising patient privacy.
Selling de-identified data removing or modifying specific identifiers that could be used to identify individuals. De-identified data retains some information that can still be useful for research and analysis, but the risk of re-identification is significantly reduced. Strict protocols are followed to ensure proper de-identification techniques are applied.
Data licensing: Rather than selling the data directly, we license it to third-party organizations or researchers. Through data licensing agreements, we provide authorized access to the data while maintaining control and ownership. We set terms and conditions regarding data usage, privacy protection, and financial arrangements.
Research partnerships: Collaborating with academic institutions, research organizations, or healthcare companies on specific research projects. These partnerships involve granting access to the data for research purposes while maintaining privacy and data protection standards. Such collaborations involve data sharing agreements and require ethical review and oversight.
Axalton Med Tech complies with applicable laws and regulations governing health data, such as the Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in the European Union. These regulations impose strict requirements on the collection, use, and disclosure of health data, including consent, security measures, and data subject rights.





